Introduction. Novel agents including Anti-CD38 monoclonal antibodies, proteasome inhibitors and immunomodulatory drugs have improved the therapeutic outcome of plasma cell dyscrasias such as multiple myeloma (MM) and systemic light chain (AL) amyloidosis. Additionally, Next-generation sequencing (NGS) has greatly improved the ability to detect genomic aberrations occurring in monoclonal plasma cells. However, NGS data has not been made use for treatment decisions of plasma cell dyscrasias. Also, mechanisms of resistance to therapeutic agents have not been clearly shown in patient samples. Moreover, in-depth differences of monoclonal plasma cells among plasma cell dyscrasias are yet to be known. In the current study, we analyzed the protein expression in monoclonal plasma cells derived from patients with MM or AL amyloidosis by mass spectrometry to understand the biological differences in cells among those diseases and changes according to treatment.
Materials and methods. Bone marrow samples from 47 newly diagnosed plasma cell dyscrasia patients (30 MM patients, 17 AL amyloidosis patients) and 9 relapsed and refractory patients diagnosed at Kumamoto University Hospital and Kumamoto Shinto General Hospital were examined. Patients provided written informed consent in accordance with the Declaration of Helsinki and the institutional ethics policy. Monoclonal plasma cells were obtained from bone marrow aspirates by CD138 magnetic beads selection. Sample preparation for proteomic analysis was performed using the PTS method, and digested peptides were desalted with SDB-XC tip. An Orbitrap Fusion Tribrid was used for peptide analysis, and peptide sequence information was acquired in data independent acquisition mode. Protein identification and quantification were performed by DIA-NN 18.1. Statistical and pathway analyses were performed using ExpressAnalyst, MetaboAnalyst and Metascape.
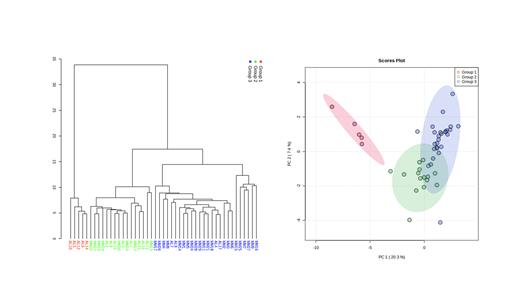
Results. Monoclonal plasma cells from plasma cell dyscrasia patients were statistically divided into 3 groups (Group 1, 2 and 3) according to protein expression. Group 1 samples had enrichment of the Endocytosis and the MAPK signaling, while samples in Group 3 were enriched with the Ribosome pathway. Group 2 seemed to have intermediate phenotype of Group 1 and 3. All the samples in Group 1 and 50% of the samples in Group 2 were from AL amyloidosis, while majority of samples in group 3 were from MM. MM cases belonging to Group 3 tend to have higher R-ISS staging and CD56 positive monoclonal plasma cells compared to Group 2 cases. Among AL amyloidosis samples, all Group 3 cases had cardiac involvement of amyloidosis, proven by significantly higher BNP expression, while Group 2 cases tend to have kidney involvement, indicated by low serum albumin. Serial analysis of samples before and after daratumumab therapy showed reduced expression of CD38 and SLAMF7 in post treatment samples. Proteins related to focal adhesion were decreased in post-daratumumab treated samples compared to pre-daratumumab samples. Samples from carfilzomib refractory patients had higher expression of cell cycle related proteins compared to their pre-treatment samples.
Conclusions. Proteomic analysis revealed differences between MM and AL amyloidosis-derived monoclonal plasma cells. While MM samples tend to belong to Group 3, which showed the activation of the Ribosome pathway, AL amyloidosis samples were mainly divided into Group 1 or 2, accompanying high enrichment of the Endocytosis and the MAPK signaling. Comparison of pre- and post-treatment samples showed distinct protein expression patterns according to therapeutic agents (daratumumab and carfilzomib). The current results may contribute to the understanding of differences between each plasma cell dyscrasias and mechanisms of resistance to therapeutic agents.
Disclosures
Kawano:Sanofi: Honoraria; Janssen Pharmaceuticals Inc: Honoraria; Ono Pharmaceutical: Honoraria; Takeda Pharmaceutical Co. Ltd.: Honoraria; Bristol Myers Squibb Co.: Honoraria; Sebia: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal